TriGen InterTAN Intramedullary Nail: A Comprehensive Overview
Orthopedic surgery has witnessed a paradigm shift in the last few years with the advent of newer technologies and implants. One such implant that has gained widespread popularity among orthopedic surgeons is the TriGen InterTAN Intramedullary Nail. In this article, we will discuss this implant, its design, indications, advantages, and disadvantages.
Introduction
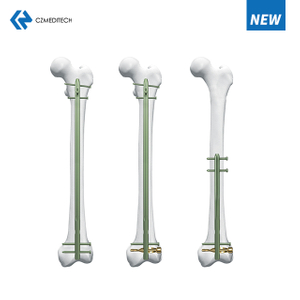
The TriGen InterTAN Intramedullary Nail is an orthopedic implant designed to provide stability and support to the femoral neck and head. This implant is used in the treatment of hip fractures, especially in elderly patients with osteoporosis. The design of this implant is based on the concept of intramedullary fixation, where the implant is inserted into the medullary canal of the bone.
Anatomy and Design
The TriGen InterTAN Intramedullary Nail is made up of titanium and has a tapered shape. The implant has three main components - the proximal body, the distal body, and the screw. The proximal body has a hook that helps in the reduction of the fracture, and the distal body has a locking mechanism that provides stability to the implant. The screw is used to compress the fracture and fix it to the implant.
Indications
The TriGen InterTAN Intramedullary Nail is primarily used in the treatment of hip fractures, including intertrochanteric and subtrochanteric fractures. The implant is also used in the treatment of periprosthetic fractures and non-unions.
Surgical Technique
The surgical technique for the TriGen InterTAN Intramedullary Nail involves the insertion of the implant into the medullary canal of the femur. The reduction of the fracture is done using the hook on the proximal body of the implant. Once the reduction is achieved, the screw is used to compress the fracture and fix it to the implant. The locking mechanism on the distal body provides stability to the implant.
Advantages
The TriGen InterTAN Intramedullary Nail has several advantages over other implants used in the treatment of hip fractures. Some of these advantages include:
Reduced surgical time and blood loss
Improved stability and fixation
Reduced risk of implant failure
Reduced risk of implant migration
Faster recovery and rehabilitation
Disadvantages
While the TriGen InterTAN Intramedullary Nail has several advantages, there are also some disadvantages associated with its use. These include:
Risk of malalignment and non-union
Risk of implant-related complications
Difficulty in removal of the implant
Complications
Like any other orthopedic implant, the TriGen InterTAN Intramedullary Nail is associated with certain complications. Some of these complications include:
Conclusion
In conclusion, the TriGen InterTAN Intramedullary Nail is a versatile implant used in the treatment of hip fractures. Its design and mechanism of action provide stability and support to the femoral neck and head, reducing the risk of implant failure and migration. While there are certain disadvantages and complications associated with its use, the TriGen InterTAN Intramedullary Nail remains a popular choice among orthopedic surgeons.
FAQs
How long does it take to recover after surgery with TriGen InterTAN Intramedullary Nail?
Ans: The recovery time varies from patient to patient and depends on various factors such as age, overall health, and severity of the fracture. However, most patients are able to
resume their daily activities within a few weeks to a few months after surgery.
Can the TriGen InterTAN Intramedullary Nail be used in young patients?
Ans: While the TriGen InterTAN Intramedullary Nail is primarily used in elderly patients with osteoporosis, it can be used in younger patients as well. However, the decision to use this implant in young patients is made on a case-by-case basis and depends on various factors such as the type and severity of the fracture.
Is removal of the TriGen InterTAN Intramedullary Nail difficult?
Ans: Removal of the TriGen InterTAN Intramedullary Nail can be difficult due to the locking mechanism on the distal body of the implant. However, with the right surgical technique, the implant can be safely and effectively removed if necessary.
How long does the TriGen InterTAN Intramedullary Nail stay in the body?
Ans: The TriGen InterTAN Intramedullary Nail is designed to remain in the body permanently. However, in some cases, the implant may need to be removed due to complications or for other reasons.
Is the TriGen InterTAN Intramedullary Nail covered by insurance?
Ans: The coverage of the TriGen InterTAN Intramedullary Nail by insurance depends on various factors such as the type of insurance and the specific coverage plan. It is recommended to check with the insurance provider for more information on coverage.